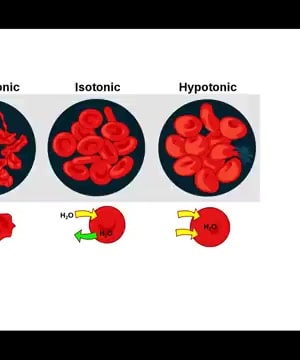
Isotonic
Sources:
Understanding Isotonic Solutions
Definition and Context
Isotonic solutions are defined by their equal concentration of solutes compared to that within cells, leading to no net movement of water across the cell membrane. This balance ensures that cells retain their shape and function optimally.
Explanation
explains that isotonic solutions maintain an equilibrium where water moves in and out of cells at equal rates. This prevents cells from shrinking in hypertonic solutions or swelling and rupturing in hypotonic solutions 1.
Practical Applications
- IV Fluids: In medical settings, intravenous fluids often use isotonic solutions to match the body's cell environment, preventing cell damage. Hypertonic solutions might be used to reduce brain swelling, while hypotonic solutions help when sodium levels are high 2.
- Sports and Hydration: For athletes, maintaining an isotonic balance is crucial for performance and recovery, ensuring cells function correctly and hydration levels are optimal 3.
Additional Uses
recommends isotonic saline solutions for nasal irrigation and gargling to reduce viral shedding due to their antibacterial properties 4. Furthermore, isotonic solutions can play a key role in adjusting the body's bioterrain swiftly during health conditions 5.
Overall, isotonic solutions are integral in various fields from medical treatments to everyday hydration and health practices, ensuring balanced and proper physiological functions.
RELATED QUESTIONS